Acid Base Titration Lab Report
To prepare a standard solution of oxalic acid. ASTM E223-08 Analysis of Sulfuric Acid.
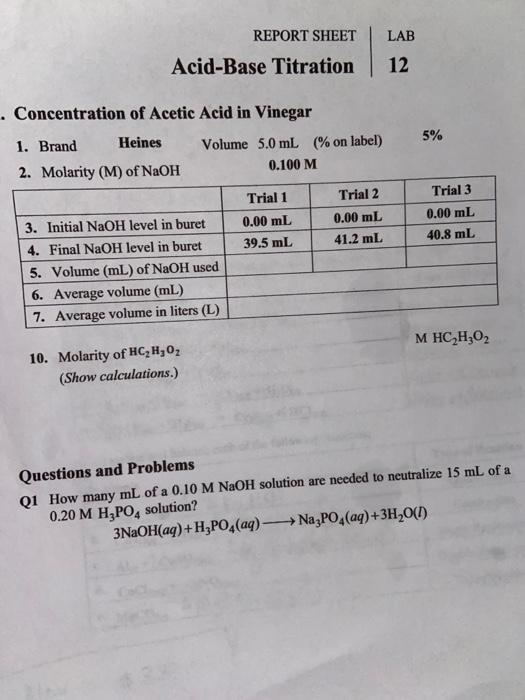
Solved Lab Report Sheet Acid Base Titration 12 Chegg Com
Standard Test Method for Acid Number of Petroleum Products by Semi-Micro Color Indicator Titration.

. Send the results to us and we will send you a report. HCl 1 365 365 H2SO4 2 981 490 CaCO3 2 100 500 AlOH3 3 780 260 For oxidationreduction reactions K is the number of moles of e- transferred per mole of oxidant or reductant in the balanced half-reaction. Get 247 customer support help when you place a homework help service order with us.
Either increases effects of the other by pharmacodynamic synergism. An example of titration usng phenolphthalein is the titration of vinegar which is technically acetic acid. STM D6174 Inorganic Sulfate in Surfactants by Potentiometric Lead Titration.
The base number is a measure of the amount of basic substance in the oil. The course emphasizes the use of experiments as a tool for chemical analysis and the reporting of results in formal lab reports. In addition to the sample an appropriate indicator is added to the titration chamber reflecting the pH range of the equivalence point.
Make sure your digital titrator is working and reset to zero. We will guide you on how to place your essay help proofreading and editing your draft fixing the grammar spelling or formatting of your paper easily and cheaply. A base is a solution that reacts with acids to form salts.
CH3COOHaq NaOHaq - CH3COONaaq H2Ol By adding the sodium hydroxide which. In the titration method phenolphthalein was used as an indicator which will be used to determine when the reaction reaches its endpoint. This experiment is designed to determine the molar concentration of acetic acid in a sample of vinegar by titrating it with a standard solution of NaOH.
Acid-base titrations depend on the neutralization between an acid and a base when mixed in solution. This experiment is to standardize the acid which is to identify its actual concentration. Introduction Vinegar is a common household item containing acetic acid as well as some other chemicals.
Standard Test Method for Base Number of Petroleum Products by Potentiometric Perchloric Acid Titration Version. To acquire the correct technique of titration. Repeat until you have added 100 digits of acid and stop.
The Mohr titration should be carried out under conditions of pH 65 9. In order to identify kissing partners that could be used as regulatory elements or building blocks of RNA scaffolds we analysed a pool of 52 10 6 RNA hairpins with randomized loops. Frooti is exported to the United States Canada the United Kingdom.
An acid dissolves in water to show acidic properties which are H ions. Hydrochloric acid is a monoprotic acid. On the report sheet record the initial reading of the NaOH solution in the buret to the nearest 002 ml.
Cement. There are two Types of Analytical Procedures first is Specifications and standard test method in Pharmacopoeias or. The course Introduction to Chemical Crystallography is intended for graduate students who wish to acquire a basic understanding of crystallography the mathematical foundations of diffraction principles the hands-on experience in the operation of X-ray diffractometers computer software for crystal structure determination and visualization as well as crystallographic databases.
It is the flagship product and most successful drink product made by Parle AgroFrooti was launched in 1985 in Tetra Pak packaging and is now also sold in PET bottles and rectangular shaped packs. Please read our Terms Conditions and Privacy Policy for information about. Indicators and colours Litmus.
ASTM D6173 Various Anionic Surfactant Actives by Potentiometric Titration. The relative amounts of these materials can be determined by titration with acids. Download Free PDF Download PDF Download Free PDF View PDF.
An Analytical Procedure is the most important key in Analytical Method ValidationThe analytical procedure defines characteristics of Drug Product or Drug Substance also gives acceptance criteria for the same. Acid-Base Titration Determination Of The Concentration Of Hydrochloric Acid Solution. It works on simple acid base titration method the free fatty acids which are present in an oil due to oxidative or hydrolytic process is titrated against a standard alkali using phenolphthalein as an indicator.
This website uses cookies to help provide you with the best possible online experience. Face-to-face Non face-to-face 1 hour 1 hour Direction. Evaluation of New Field Test Methods for Base Number and Acid Number in Lubricating Fluids Dexsil.
Frooti is a mango-flavoured drink sold in IndiaIt is made with natural flavours and mango-concentrate. To calculate the concentration of HCl solution in an acid-base titration Student-Learning Time. Get Smart with Improved TAN Titrations.
The amount of alkali consumed represents the amount of. To explain the precautions required during titration vi. As more base is added to the titrands solution the pH changes becoming more basic and the solution changes color.
PH 32 red pH pH 44 yellow. Topics include the quantitative analysis of a multicomponent mixture using complexation and double endpoint titration pH measurement buffers and pH indicators the kinetic study of a redox reaction and the. Ask your instructor to check your reading and initial your report.
PH 6 red pH 7 purple pH 8 blue Methyl orange. Download Free PDF Download PDF Download Free PDF View PDF. To determine the concentration of HCl solution.
Usually with this indicator when the titrands solution just starts to turn pink you have reached the endpoint. This experiment uses the titration method. Then titrate 1000 ml of deionized water with your 016N acid as follows.
ASTM Intl West Conshohocken Pa. Get Involved MEMBERSHIP. 3531 Tests for acid-base indicators See.
Balanced half reaction K Fe3 Æ Fe 3 I2 Æ 2 I-2 2 S2O3 2-Æ S 4O6 2-1 Using Normality in titration calculations. The stability of kissing complexes relies on loop parameters base composition sequence and size and base combination at the looploop helix - stem junctions. Acid-base indicators change colour in acidic or basic solutions.
Read over the lab manual and then answer the following question. Sodium Carbonate in the other hand is a base. They may be weak acids that dissociate and change colour in alkaline solutions.
CHE 226 Analytical Chemistry Laboratory 40 Acid-Base Titration EXPERIMENT 7 Identifying a Substance by Acid-Base Titration SAFETY WARNING. To standardise 02 M NaOH solution. Student LAB MANUAL Autonomous 2016-by mantena raju.
At higher pH silver ions may be removed by precipitation with hydroxide ions and at low pH chromate ions may be removed by an acid-base reaction to form hydrogen chromate ions or dichromate ions affecting the accuracy of the end point. In a titration it is critical to know the exact concentration of NaOH in order to determine the concentration of the solution being tested. ASTM D6209-13 Test to determine Gaseous and Particulate Polycyclic Aromatic Hydrocarbons in Ambient Air.
Patients should report promptly any unexplained muscle pain tenderness or weakness particularly if accompanied by. ASTM D6238-13 Test for Total Oxygen Demand in Water. KHP is a weak acid and reacts with base in the following way.
Add 10 digits of acid record digits and pH increase acid to 20 digits record pH. After initiation or upon titration analyze lipid levels within 2-4 weeks and adjust dosage accordingly. Place a white sheet of paper under Erlenmeyer flask 1 to facilitate the detection of the end point when noting the color change of the indicator.

Solved Report Sheet Acid Base Titration Lab 10 M A Chegg Com

Acid Base Titration Lab Report Great College Essay

Solved Section Titration Lab Report Sheet Table 1 Acid Base Chegg Com

0 Response to "Acid Base Titration Lab Report"
Post a Comment